New computational framework to understand aggressive cancer cell behavior
An interdisciplinary team of scientists at U-M has formulated a new conceptual approach to understanding the conditions that motivate cancer cells to collaborate. The team, including CSE assistant professor Nikola Banovic, is working to overcome the tremendous variability that exists in the progression of the disease by building large, single-cell data sets for reinforcement learning models. The effort has been awarded a $1M grant from the W. M. Keck Foundation.
Cancer is an illness caused by an uncontrolled division of transformed cells, which can originate in almost any organ of the body. Cancer is not a single disease, even when it arises in the same site of the body. Tremendous variability exists in progression of disease and response to therapy among different persons with the same general type of cancer, such as breast cancer. Even at the level of a single person, cancer cells show tremendous heterogeneity within a single tumor and among a primary tumor and metastases.
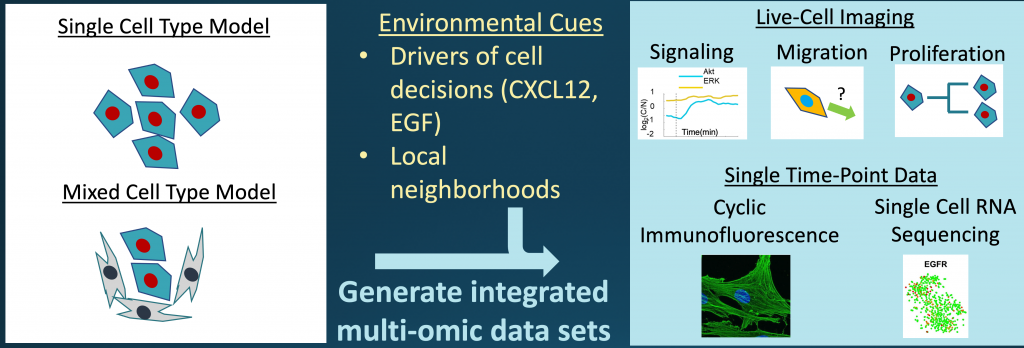
This heterogeneity causes drug resistance and fatal disease. The prevailing dogma is that heterogeneity among cancer cells arises randomly, generating greedy individual cancer cells that compete for growth factors and optimal environments. The rare “winners” in this competition survive and metastasize. However, tumors consistently maintain heterogeneous subpopulations of cancer cells, some of which appear less able to grow and spread. This observation prompted Gary and Kathy Luker, U-M cancer cell biologists, to hypothesize that cancer cells may actually collaborate under some circumstances to cause disease and not just compete. The idea that single, heterogeneous cancer cells work collectively within a constrained range of variability to drive population-level outputs in tumor progression is a ground-breaking concept that may revolutionize how we approach cancer biology and therapy.
The cancer cell biologists have teamed up with computational scientists and experts in artificial intelligence to focus the power of these fields on understanding and overcoming heterogeneity in cancer. Building on large, single-cell data sets unique to the team, they will combine inverse reinforcement learning, an artificial intelligence method typically applied to discover motivations for human behaviors, with computational models inferred on the basis of the physics and chemistry of cell signaling and migration. They have proposed an entirely new conceptual approach combining single cell data, physics-based modeling and artificial intelligence to single-cell heterogeneity and intercellular interactions. By discovering testable molecular processes underlying “decision-making” by single cells and their “motivations” for acting competitively or collaboratively, this research blazes a new path to understand and treat cancer.
The team includes Gary Luker (Radiology, Microbiology and Immunology; Biomedical Engineering), and Kathryn Luker (Radiology), who are leading the experimental studies of cell signaling and migration; Jennifer Linderman (Chemical Engineering; Biomedical Engineering); and Krishna Garikipati (Mechanical Engineering; Mathematics), who are leading the machine learning and modeling side of the project. Nikola Banovic (CSE) and Xun Huan (Mechanical Engineering) are using artificial intelligence approaches to discover decision-making policies and rewards for cancer cells, working with the rest of the investigators to incorporate experimental data and physics/chemistry-based models into their approaches.
The idea for this project originated in the 2020 MICDE faculty workshop in AI for Physically based Bio-medicine Workshop. The workshop brought together an interdisciplinary group of faculty members to discuss ways to advance artificial intelligence and machine learning methods for biomedical problems. After seeding the idea, a subset of these researchers were awarded an MICDE catalyst grant and a MIDAS PODS grant. These funds were used to establish the proof of concept and to generate preliminary results.
While the current proposal focuses on cancer, this innovative computational framework represents a transformative leap with widespread applications in multiple other biomedical, physical, and social sciences.

This article was written by Mariana Carrasco-Teja for MICDE.
 MENU
MENU 
